Welcome to a deep dive into the fascinating world of autophagy, the body’s natural recycling system that plays a crucial role in maintaining cellular health and longevity. Join me, Dr. Ben Bikman, as we explore how this vital process works, its connection to metabolic health, and how you can leverage it for a longer, healthier life.
What is Autophagy?
Autophagy, derived from the Greek term meaning “self-eating,” is a critical biological process where cells degrade and recycle their own components. This self-renewal mechanism is essential for cellular homeostasis, allowing cells to maintain their function and integrity. By breaking down damaged organelles and misfolded proteins, autophagy ensures that cells can adapt to stress and nutrient deprivation.
This process is not merely a cleanup operation; it’s a sophisticated system that enables cells to repurpose their components. When cellular components are broken down, the resulting building blocks can be reused to synthesize new proteins or generate energy, making autophagy a vital aspect of cellular metabolism.
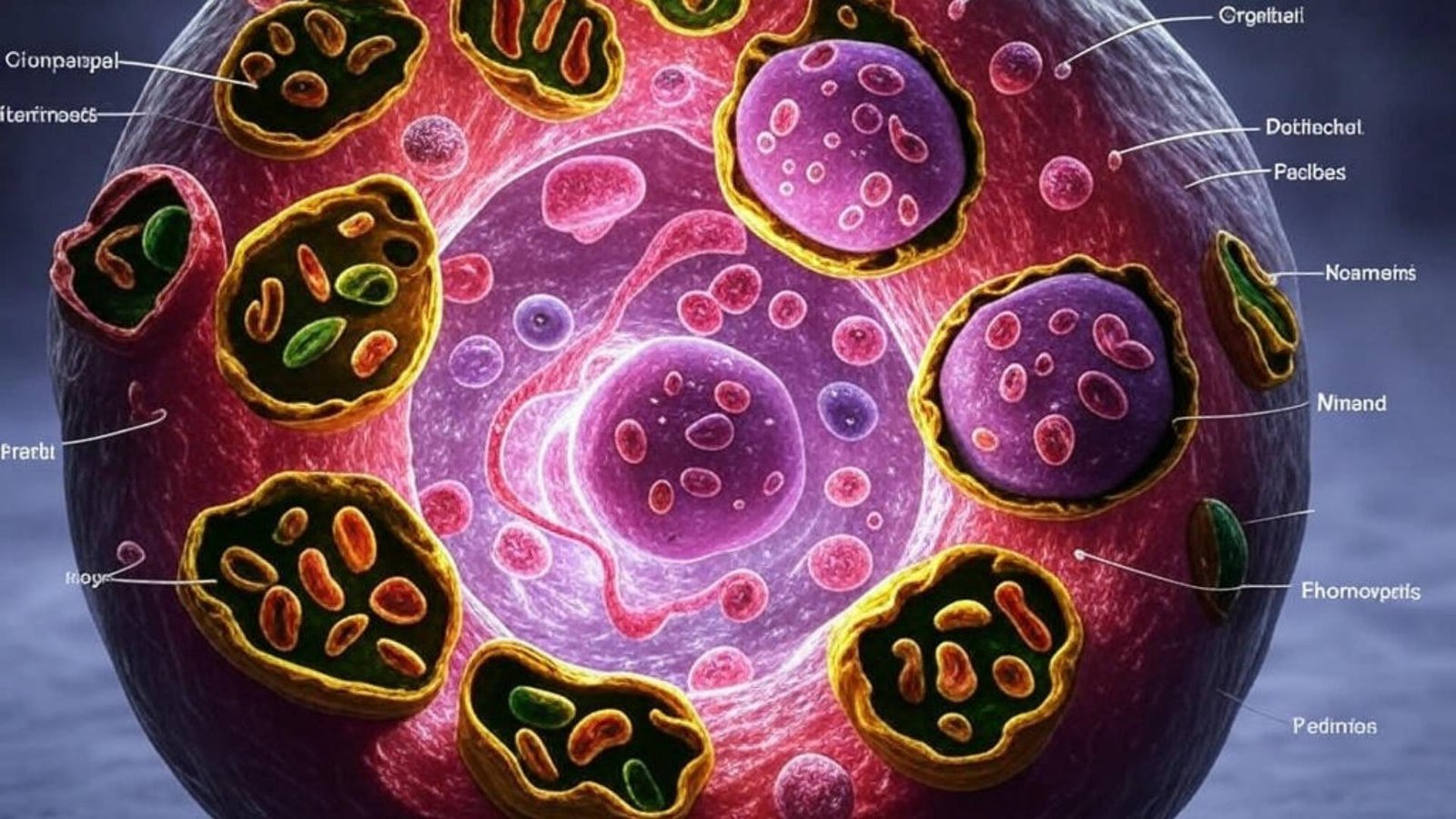
Understanding Organelles and Their Role
Organelles are specialized structures within cells that perform distinct functions crucial for cellular life. Key organelles include:
- Nucleus: The control center containing genetic material.
- Mitochondria: The powerhouse responsible for energy production.
- Endoplasmic Reticulum: Involved in protein and lipid synthesis.
- Lysosomes: The cell’s recycling center, breaking down waste materials.
Over time, organelles can become damaged or dysfunctional. Autophagy plays a pivotal role in identifying and replacing these damaged components, thereby preventing cellular dysfunction. By efficiently managing organelle turnover, cells can maintain optimal performance and longevity.
The Lysosome: The Cell’s Recycling Center
The lysosome is often termed the cell’s recycling center for good reason. This organelle contains a variety of enzymes that can break down different types of biomolecules, including proteins, lipids, and carbohydrates. When autophagy is activated, damaged organelles or proteins are sequestered into autophagosomes, which then fuse with lysosomes. This fusion allows lysosomal enzymes to degrade the contents, recycling the resultant molecules back into the cytoplasm.
The recycling process is crucial for cellular health. By removing waste and recycling components, lysosomes help maintain cellular balance and prevent the accumulation of harmful substances. This process is particularly important during periods of stress, such as nutrient deprivation, where the cell must rely on internal resources to survive.
Autophagy and Cellular Quality Control
Cellular quality control is one of the most significant roles of autophagy. By degrading damaged organelles and misfolded proteins, autophagy prevents the accumulation of cellular debris that can impair cell function. This process is essential for maintaining cellular integrity and preventing diseases associated with aging and metabolic disorders.
Research has shown that impaired autophagy is linked to various neurodegenerative diseases, such as Alzheimer’s and Parkinson’s. In these conditions, the inability to clear misfolded proteins leads to toxicity and cellular dysfunction. Enhancing autophagic activity may provide a therapeutic strategy for mitigating these diseases by promoting cellular cleanup and restoration.
Energy Production During Nutrient Deprivation
During periods of nutrient deprivation, such as fasting, autophagy becomes a lifeline for cells. It provides essential building blocks and energy through the breakdown of cellular components. This process ensures that cells can continue to function effectively, even in the absence of external nutrients.
When the body is deprived of food, autophagy is upregulated to release amino acids and fatty acids from degraded organelles. These molecules can be utilized in various metabolic pathways to generate ATP, the energy currency of the cell. This self-sustaining mechanism is vital for survival during fasting or starvation, underscoring the importance of autophagy in cellular energy management.
Moreover, the breakdown of lipids during autophagy can lead to the production of ketones, which serve as an alternative energy source for many tissues, particularly the brain. This adaptability highlights the significance of autophagy not only in maintaining cellular health but also in ensuring survival during challenging metabolic states.
Preventing Diseases Through Autophagy
Autophagy plays a pivotal role in disease prevention by maintaining cellular health. This process is especially crucial in combating neurodegenerative diseases, such as Alzheimer’s and Parkinson’s. Accumulation of damaged proteins and organelles can lead to cellular dysfunction, and autophagy acts as a safeguard against this deterioration.
Research indicates that enhancing autophagic activity can mitigate the effects of these diseases. By promoting the clearance of toxic aggregates and misfolded proteins, autophagy helps maintain neuronal health and function. Furthermore, in conditions like atherosclerosis, autophagy can help manage lipid metabolism, reducing the risk of cardiovascular complications.
Mechanisms of Disease Prevention
- Protein Quality Control: Autophagy degrades misfolded proteins, preventing their accumulation that can cause cellular toxicity.
- Organellar Maintenance: By removing damaged organelles, autophagy ensures that cells operate efficiently, reducing the risk of cellular aging.
- Inflammation Reduction: Enhanced autophagy can lower inflammation levels, a key factor in many chronic diseases.
In essence, autophagy serves as a cellular defense mechanism, preserving health and preventing the onset of various diseases associated with aging and metabolic dysfunction.
The Connection Between Autophagy and Obesity
Obesity is not merely a result of excessive calorie intake; it is intricately linked to autophagic processes within adipose tissue. When fat cells become enlarged, their autophagic activity can be impaired, leading to a cascade of metabolic dysfunctions.
Studies have shown that reduced autophagic function in adipocytes correlates with increased inflammation, insulin resistance, and other metabolic disorders. In obese individuals, the accumulation of dysfunctional fat can exacerbate these issues, creating a vicious cycle of inflammation and metabolic decline.
Autophagy’s Role in Adipose Tissue Health
- Inflammation Control: Adequate autophagy can help mitigate the inflammatory response in hypertrophic adipose tissue.
- Insulin Sensitivity: Restoration of autophagic activity has been linked to improved insulin sensitivity, crucial for metabolic health.
- Fat Cell Functionality: Maintaining autophagy in fat cells can enhance their ability to store and mobilize fats effectively.
In summary, boosting autophagic activity in adipose tissues may serve as a therapeutic strategy to combat obesity and its related metabolic complications.
Emerging Evidence in Human Studies
While much of the research on autophagy has been conducted in animal models, emerging studies in humans are beginning to shed light on its significance. Observational studies indicate that autophagic markers in adipose tissue differ between obese and lean individuals, suggesting a potential link to metabolic health.
For instance, research has identified elevated levels of autophagy-related proteins in visceral fat of obese participants, indicating that autophagy is active but potentially dysregulated in response to excess fat. This dysregulation may contribute to the inflammation and metabolic dysfunction commonly seen in obesity.
Key Findings from Human Studies
- Altered Autophagic Markers: Studies have shown differences in levels of autophagy markers, such as LC3 and ATG5, between obese and lean individuals.
- Visceral Fat Dynamics: Increased autophagy in visceral fat may be a compensatory response to manage the stress of excess fat accumulation.
- Potential Therapeutic Targets: Understanding these changes could inform future therapies aimed at enhancing autophagy to improve metabolic health.
These findings underscore the importance of investigating autophagy in human populations to establish its role in obesity and metabolic disorders.
Exploring Autophagy’s Role in Longevity
Autophagy is emerging as a key player in the quest for longevity. Its ability to clear damaged cellular components not only promotes cellular health but also has implications for extending lifespan. As autophagic activity declines with age, the accumulation of cellular debris can lead to various age-related diseases.
Research has demonstrated that enhancing autophagy can improve healthspan and potentially extend lifespan across various organisms. For example, genetic studies in mice have shown that increased autophagic activity correlates with improved health markers and extended longevity.
Longevity Mechanisms Associated with Autophagy
- Cellular Cleanup: Regular autophagic activity prevents the accumulation of damaged proteins and organelles, essential for maintaining youthful cellular function.
- Stress Resistance: Enhanced autophagy can improve the cell’s ability to adapt to stressors, further contributing to longevity.
- Metabolic Health: By promoting insulin sensitivity and reducing inflammation, autophagy supports overall metabolic health, a critical component of longevity.
In essence, fostering autophagic processes may be a viable strategy for enhancing healthspan and longevity.
Pharmacological Interventions: The Case of Rapamycin
Rapamycin, a well-known mTOR inhibitor, has garnered attention for its potential to enhance autophagic activity. By inhibiting mTOR, rapamycin promotes the breakdown of cellular components, which can lead to improved health outcomes and extended lifespan in animal models.
However, the use of rapamycin is not without its challenges. While it shows promise, it also carries potential side effects, particularly concerning immune function and muscle recovery. This raises important questions about the balance of autophagy and anabolic processes in human health.
Considerations for Rapamycin Use
- Immune Function: Chronic use of rapamycin can compromise the immune system, necessitating caution in its application.
- Muscle Health: Evidence suggests that rapamycin may inhibit muscle protein synthesis, impacting recovery and growth.
- Individual Variability: The effects of rapamycin may vary significantly among individuals, emphasizing the need for personalized approaches to its use.
While rapamycin offers intriguing possibilities for enhancing autophagy and longevity, careful consideration of its effects is essential for safe and effective use.
The Role of Insulin in Regulating Autophagy
Insulin is a key player in the regulation of autophagy. As a hormone, it signals the body’s nutrient status, primarily indicating when food is abundant. When insulin levels rise, it promotes an anabolic state, encouraging growth, repair, and storage. This is essential for immediate energy needs but can inhibit autophagy, the body’s catabolic process.
In essence, high insulin levels signal the body to prioritize building processes over cleaning processes. This means that when insulin is persistently elevated, autophagy can be suppressed, leading to the accumulation of damaged cellular components. This accumulation can contribute to cellular dysfunction and various metabolic disorders.
Understanding this balance between insulin and autophagy is crucial for maintaining metabolic health. Periodic fluctuations in insulin levels, such as those induced by fasting or a low-carb diet, can reactivate autophagy, allowing for cellular cleanup and regeneration.
How Low-Carb and Ketogenic Diets Enhance Autophagy
Low-carb and ketogenic diets are known for their ability to lower insulin levels significantly. When carbohydrate intake is reduced, insulin secretion decreases, creating an environment conducive to autophagy. This shift allows the body to tap into its fat stores for energy, promoting fat oxidation and ketogenesis.
Research indicates that ketogenic diets can enhance autophagic activity in various tissues, including the brain. By lowering insulin, these diets not only activate autophagy but also increase the production of ketones, which can directly stimulate autophagic pathways.
Moreover, the ketone body beta-hydroxybutyrate (BHB) plays a vital role in this process. BHB not only enhances autophagy but also improves the clearance of misfolded proteins, making it particularly beneficial for neurodegenerative conditions. This dual action underscores the potential of ketogenic diets in promoting cellular health and longevity.
Balancing Anabolism and Catabolism
Achieving a balance between anabolic and catabolic processes is essential for optimal health. While autophagy is crucial for cellular maintenance, excessive catabolism can lead to muscle wasting and other negative outcomes. Therefore, it is vital to ensure that periods of autophagy are balanced with times of growth and repair.
Protein intake plays a significant role in this balance. Consuming adequate protein can stimulate insulin and activate mTOR, promoting anabolic activities while still allowing for autophagic processes during periods of low insulin, such as fasting or low-carb eating. This strategic approach enables the body to maintain muscle mass while benefiting from the cellular cleanup that autophagy provides.
In summary, the interplay between insulin, autophagy, and dietary choices highlights the importance of a well-rounded approach to nutrition. By understanding how to manipulate these signals, we can optimize our metabolic health and longevity.
Conclusion: Harnessing Nutritional Signals for Autophagy
Autophagy is a vital process for maintaining cellular health and preventing age-related diseases. By understanding the role of insulin and the impact of dietary choices, we can leverage nutritional signals to enhance autophagic activity. Low-carb and ketogenic diets offer promising avenues for promoting autophagy, particularly in the context of neurodegenerative diseases and metabolic disorders.
To harness the benefits of autophagy, it is essential to adopt a balanced approach that incorporates periods of both catabolism and anabolism. By strategically managing insulin levels through dietary choices, we can optimize autophagy for improved health and longevity.
Ultimately, understanding and manipulating these metabolic pathways empowers us to take charge of our health, paving the way for a longer, healthier life.
FAQs About Autophagy
What is autophagy?
Autophagy is the process by which cells degrade and recycle their own components, ensuring cellular health and function.
How does insulin affect autophagy?
Insulin promotes anabolic processes and inhibits autophagy. Elevated insulin levels can suppress autophagic activity, leading to cellular dysfunction.
Can diet influence autophagy?
Yes, diets low in carbohydrates, such as ketogenic diets, can enhance autophagy by lowering insulin levels and promoting the production of ketones.
What role do ketones play in autophagy?
Ketones, particularly beta-hydroxybutyrate (BHB), can stimulate autophagy and aid in the clearance of misfolded proteins, making them beneficial for brain health.
Is autophagy beneficial for longevity?
Yes, enhancing autophagy has been linked to improved healthspan and longevity by preventing the accumulation of damaged cellular components.